The inner workings of small beings
The video above is more Mesolens magic. Here, we’re looking through the tip of the pseudoscorpion’s pedipalp to see the venom apparatus and the surrounding tissues. Video generated by Gail McConnell and the Mesolens lab on specimens collected by David Wilcockson and I.
(Much of this text is by Prof Gail McConnell, with a few additions from me)
Most animals are very small, less than 10 mm, but this small size belies incredible structural complexity. How do you go about studying the intricate inner workings of these tiny beings? Dissection is possible, but very destructive as cutting into the specimen destroys many delicate structures. You could section it by chopping it into lots of vanishingly thin slices, but this is laborious, fraught with difficulties and the specimen is still being cut, thus destroying the overall structure.
Advances in imaging now mean that you don’t have to cut into or destroy the specimen to see its inner workings. Micro-CT uses x-rays to see inside organisms that are a few centimetres in diameter; however, the spatial resolution is poor. With this technique you can see detail at the organ level, but it’s not really possible to see individual cells, let alone sub-cellular detail.
A standard confocal microscope will allow you to see sub-cellular detail, but normally only in very tiny specimens, or small sub-regions of a specimen. This is because of the historical relationship of the ratio of magnification to numerical aperture (collection angle), which arose because the light microscope was designed with the human eye as the detector, and there was no reason to provide more spatial detail than the eye could see.
Nowadays, however, we hardly use the human eye in light microscopy. We normally have a cursory glance at the object we want to study using the binocular viewer, but then switch to a camera or other sensitive photodetector to make a digital image (as opposed to the historical method of looking down the eyepieces and then sketching the viewed object by hand). The manufacturers of microscopes have been slow to develop new lenses that can give higher spatial resolution images over large volumes. This is because such a lens would be too large to fit on any existing microscope, and so it would be too much of a business risk to develop something totally new. But that is precisely what researchers at the University of Strathclyde have done because they understood the need for such an instrument in science.
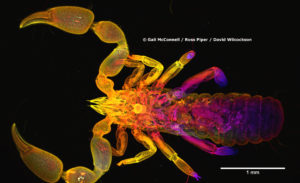
Their invention, the Mesolens, is a revolutionary light microscope that enables you to see sub-cellular detail throughout large specimens without dissection. We used the Mesolens to peer inside the pseudoscorpion you can see here. David Wilcockson and I collected these animals in darkest Peru and preserved them in a mixture of glacial acetic acid and 60% ethanol. To get this mixture into all the tissues of the pseudoscorpions we very carefully pierced the soft abdomen with a very fine tungsten needle. On our return to the UK, the specimens were sent to Gail McConnell – one of the inventors of the Mesolens. Gail and her colleagues added a fluorescent dye to the specimens to make the nucleus of each cell fluorescent, and then it was treated with a series of chemicals to make it transparent, so we can see interior detail like muscle through the normally opaque exoskeleton. A laser was scanned through the entire specimen, and where the laser interacted with the dye it resulted in the emission of a fluorescent light. This light was collected from the stained regions by a photodetector and was used to make the digital image. This image is hugely compressed, and the very fine detail is not visible at this level of magnification: to see individual nuclei, it is necessary to digitally zoom into the dataset. The full dataset is over 20GB and comprises 180 different images taken at different depths within the specimen. These images have been assigned a different colour for display here: white is the near-side (e.g. dorsal), orange is the middle of the specimen, and blue is the far-side (e.g. ventral).
A standard confocal microscope with a 4x lens (the same magnification as the Mesolens) would allow study of the same 118 cubic millimetre volume, but the resolution is 5 times poorer in the xy (lateral) plane, and 25 times worse in the z (axial) plane. As such, a standard confocal microscope with a low power lens doesn’t let you see inside cells. The Mesolens, which the inventors have developed as either a camera lens or which can be used with a scanned laser beam the same way as a standard confocal microscope (but with giant mirrors and many more pixels in the images, hence the huge datasets) has sub-cellular resolution throughout this large volume, allowing the study of large specimens such as whole Drosophila, Tribolium, Culex, and now our pseudoscorpions!
This collaboration with Gail and her colleagues is part of our work to understand the inner workings of these animals, especially their venom apparatus.
The whole, explorable 20 GB dataset will be available when we publish this work. Stay tuned for more.
Some links to take a look at:
Prof Gail McConnell: https://www.strath.ac.uk/staff/mcconnellgailprof/
The Mesolens:
http://www.strathclydemesolab.com/
Leave a Reply